REDUCED ADHESION OF AGED INTESTINAL STEM CELLS CONTRIBUTES TO AN ACCLERATED CLONAL DRIFT
by Ali Hageb, Torsten Thalheim, Jörg Galle, Hartmut Geiger, et al
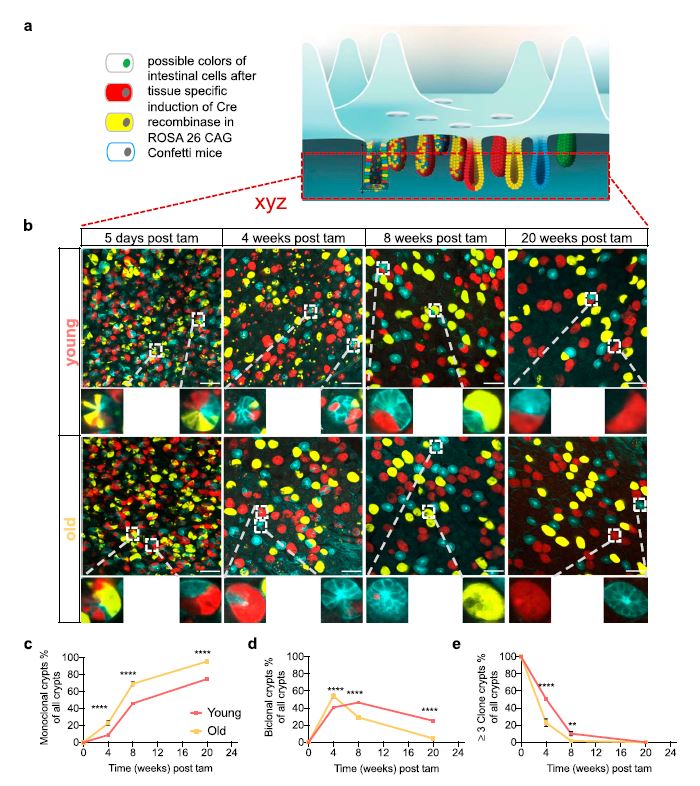
Upon aging, the function of the intestinal epithelium declines with a concomitant increase in aging-related diseases. ISCs play an important role in this process. It is known that ISC clonal dynamics follow a neutral drift model. However, it is not clear whether the drift model is still valid in aged ISCs. Tracking of clonal dynamics by clonal tracing revealed that aged crypts drift into monoclonality substantially faster than young ones. However, ISC tracing experiments, in vivo and ex vivo, implied a similar clonal expansion ability of both young and aged ISCs. Single-cell RNA sequencing for 1,920 high Lgr5 ISCs from young
and aged mice revealed increased heterogeneity among subgroups of aged ISCs. Genes associated with cell adhesion were down-regulated in aged ISCs. ISCs of aged mice indeed show weaker adhesion to the matrix. Simulations applying a single cell–based model of the small intestinal crypt demonstrated an
accelerated clonal drift at reduced adhesion strength, implying a central role for reduced adhesion for affecting clonal dynamics upon aging.
INTRODUCTION
A hallmark of the intestine is cup-shaped crypts harboring intestinal stem cells (ISCs). ISCs are located at the base of the intestinal crypt, flanked by differentiated Paneth cells, followed by a highly proliferating transient amplifying (TA) progenitor cell zone. TA cells further proliferate and differentiate into enterocytes, goblet cells, and enteroendocrine cells that migrate out of the crypt to form the finger-like villi. ISCs replace the intestinal epithelium every 3–5 d to maintain intestinal tissue homeostasis and to supply the intestinal epithelium with TA cells (Barker, 2014). ISCs express the marker protein Lgr5 (Barker et al, 2007; Sato et al, 2009). There are between 12 and 16 Lgr5 expressing cells per crypt. However, only 5–7 out these might be functionally active ISCs (Kozar et al, 2013). Intestinal homeostasis in young animals goes along with a clonal competition among ISCs that can be described by a model of neutral drift or neutral competition (Lopez-Garcia et al, 2010; Snippert et al, 2010; Grun et al, 2015). In this model, individual ISCs in a crypt are almost vequipotent but stochastically and continuously acquire the ability
to dominate the generation of differentiated cells to the villus for a defined amount of time, while being replaced by clonal expansion of other ISCs that will subsequently dominate contribution to the villus and so on (Snippert et al, 2010). Changes of the competition dynamics have been investigated in mice (Snippert et al, 2014; Huels et al, 2018) and by applying single cell–based computational models (Thalheim et al, 2016). In young mice, reports support that in 7–8 wk, between 50% and up to 75–80% of all crypts turn monoclonal, whereas it might take up to 30 wk to turn all crypts monoclonal (Winton & Ponder, 1990; Li et al, 1994; Lopez-Garcia et al, 2010; Snippert et al, 2010). During the next couple of weeks, one novel, again almost equipotent neutral ISC subclone within the currently monoclonal crypt, will replace the current dominant ISC clone to turn the crypt again monoclonal.
Aging results in a decline of the function of ISCs. This decline includes reduced self-renewal potential and the ability to maintain tissue homeostasis upon stress (Martin et al, 1998; Potten et al, 2001). For example, crypts or ISCs from aged mice present with a reduced percentage of cells that are able to form fully structured organoids in ex vivo assays (Nalapareddy et al, 2017, 2021; Mihaylova et al, 2018; Pentinmikko et al, 2019). Aging of ISCs is thought to be amain driver of aging-related diseases of the digestive track, like malabsorption or cancer (Saffrey, 2014; Jasper, 2020). ISCs are, over the lifespan of the organism, exposed to challenging environments like acids, enzymes, nutrition elements, microbial pathogens, and microbiome composition. Such environmental factors might be a driving force in aging of ISCs. In addition, signals from Paneth cells that form a niche for ISCs have been reported to play a role in diminishing the stemness potential of aged ISCs. For example, Notum, a Wnt antagonist that is primarily
produced by Paneth cells, attenuates ISC-driven regeneration of aged intestinal epithelium (Pentinmikko et al, 2019). Although there is a growing understanding of the molecular mechanisms that underlie aging of ISCs, a more detailed understanding on how aging affects ISC potential and clonal dynamics in vivo is missing. For example, a current paradigm in the hematopoietic system is that there is an increase in heterogeneity among stem cells upon aging. This increase seems to be linked to a lower number of clones (in this case dominant) that contribute actively to blood cell formation, which has been termed clonal hematopoiesis. It is thought that these changes are causal for unwanted agingassociated diseases of the hematopoietic system (Akunuru & Geiger, 2016). ScRNA-seq analyses of young ISCs showed an initially homogenous pool of ISCs (Grun et al, 2015), which however could be further subdivided into two or three subgroups by singlecell expression profiling, based on the intestinal region ISCs were derived from but more importantly, according to their ability to interact with distinct signals from the environment (Haber et al, 2017; Biton et al, 2018). The level of heterogeneity among aged ISCs is not known. Novel knowledge on changes among individual ISCs upon aging will help to further understand consequences of aging of ISCs for tissue homeostasis and likely also for aging-associated
clonal outgrowth like in cancer…
click here to read the full article: https://www.life-science-alliance.org/content/5/8/e202201408