TRANSCRIPTIONAL STATES OF CAR-T INFUSION RELATE TO NEUROTOXICITY – LESSONS FROM HIGH-RESOLUTION SINGLE-CELL SOM EXPRESSION PORTRAYING
written by Henry Loeffler-Wirth, Michael Rade, Arsen Arakelyan, Markus Kreuz, Markus Loeffler, Ulrike Koehl, Kristin Reiche and Hans Binder
Anti-CD19 CAR-T cell immunotherapy is a hopeful treatment option for patients with B cell lymphomas, however it copes with partly severe adverse effects like neurotoxicity. Single-cell resolved molecular data sets in combination with clinical parametrization allow for comprehensive characterization of cellular subpopulations, their transcriptomic states, and their relation to the adverse effects. We here present a re-analysis of single-cell RNA sequencing data of 24 patients comprising more than 130,000 cells with focus on cellular states and their association to immune cell related neurotoxicity. For this, we developed a single-cell data portraying workflow to disentangle the transcriptional state space with single-cell resolution and its analysis in terms of modularly-composed cellular programs. We demonstrated capabilities of single-cell data portraying to disentangle transcriptional states using intuitive visualization, functional mining, molecular cell stratification, and variability analyses. Our analysis revealed that the T cell composition of the patient’s infusion product as well as the spectrum of their transcriptional states of cells derived from patients with low ICANS grade do not markedly differ from those of cells from high ICANS patients, while the relative abundancies, particularly that of cycling cells, of LAG3-mediated exhaustion and of CAR positive cells, vary. Our study provides molecular details of the transcriptomic landscape with possible impact to overcome neurotoxicity.
INTRODUCTION
Chimeric Antigen Receptor (CAR)-T cell therapy is a promising treatment option for patients with B cell lymphomas. It belongs to the family of immunotherapies which share the concept of directing the patients’ immune system against the tumor by releasing breaks of immune regulation and suppression (immune checkpoint inhibition), by boosting an immune response using signaling molecules (cytokine therapy), or by infusion of adapted immune cells to optimally target and dispose the malignant cells (cellular immunotherapy, tumor-infiltrating lymphocytes, and cancer vaccine therapy). Specialized T cells with CAR have proven efficacy in therapy of diverse hematopoietic and lymphatic malignancies such as leukemia, myelomas and lymphomas. CAR-T cells are characterized by receptor proteins that have been engineered to target binding partners as specific as possible for a particular disease, for example CD19 for therapy of B cell lymphoma. Until now, three generations of receptor proteins have been developed, incrementally optimizing the signaling domain of the receptor construct.
However, with increasing efficacy of the therapies, immune responses unleashed by immunotherapies cause a series of adverse outcome effects, particularly the cytokine release syndrome (CRS) and neurotoxicity (immune effector cell-associated neurotoxicity syndrome – ICANS) often accompany CAR-T cell therapies, rendering risk estimation prior to therapy, safety monitoring and adjunctive treatments indispensable for a save cancer therapy. As part of the project imSAVAR (Immune Safety Avatar: nonclinical mimicking of the immune system effects of immunomodulatory therapies; www.imsavar.eu), we aim at identification of potential markers, understanding underlying mechanisms and, in perspective, at providing prediction models for adverse effects of immunomodulatory therapeutics.
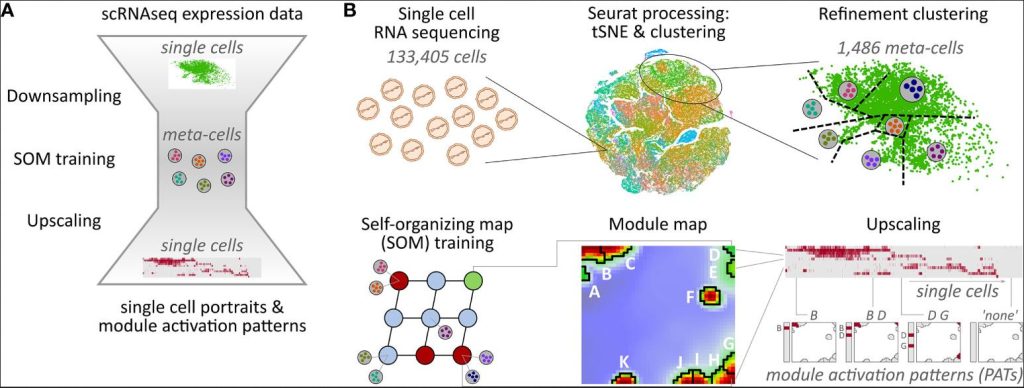
Recent studies applied single-cell RNA sequencing to analyses the transcriptome of the infusion products and/or blood samples of patients undergoing anti-CD19 CAR-T cell therapy. The data set published by Deng et al., 2020 contains 24 infusion product transcriptomes along with information about adverse effects. Their results suggest that heterogeneity in the cellular and molecular features of CAR-T cell infusion products contributes to variation in efficacy and toxicity after therapy of lymphomas. In our re-analysis of this data, we extend the original analyses by an in-detail characterization of the expression landscapes with single-cell resolution and with special focus on T cell subpopulation composition, their transcriptional states, and their relation to neurotoxic side effects. For this, we developed the so-called single-cell data portraying approach by adapting and extending our previous work on self-organizing maps (SOM) portraying of transcriptome, methylome and genome data in bulk settings, and its application in the context of cancer, inflammatory diseases, and health research. The method is based on SOM machine learning, and supplements it with extensive analysis and visualization options including pseudo-temporal ordering, combined omics studies, and proof-of-principle applications in single-cell experiments. It is available as R-package ‘oposSOM’ on Bioconductor and GitHub repositories, and part of the results of the previous studies are available in an interactive data and results browsing tool.
In this publication, we present comprehensive data portraying of the infusion products of CAR-T cell immunotherapy in order to decipher transcriptomic landscapes in terms of defined activation patterns related to the different T cell subpopulations, cellular functions, variations as a function of the CAR construct, and neurotoxicity complications. We will demonstrate capabilities of the single-cell data portraying to disentangle complex co-expression relations of the genes as well as the broad similarity and variability continuum of more than hundred thousand cells. Our analysis revealed that the transcriptional states of cells derived from patients with low ICANS grade do not differ from those of cells from high ICANS patients. On the other hand, their relative abundancies vary markedly thus making relative frequencies of distinct cell states a potential indicator for adverse effects.
…
CONCLUSION
We provided a comprehensive single cell data portraying and demonstrated its capabilities to disentangle transcriptional states using intuitive visualization, functional mining, molecular cell stratification, and variability analyses. We have shown that scalable resolution from single-cell- to subpopulation-level generates novel insights into high-dimensional data sets, and particularly of CAR-T infusion products which extend the results reported in the original publication: We find that the transcriptional states of cells derived from patients with low ICANS grade do not differ from those of cells from high ICANS patients, while their relative abundancies with regard to cell cycle activity, CAR status and T cell exhaustion vary markedly.
Our results indicate that therapies can be improved by depletion of cell cycle arrested and CAR- cells, however CAR-T cell immunotherapy optimization needs further studies with higher number of patients involved to monitor a broader range of cellular and transcriptional states.
to read the full article at frontiers online magazine, please click here: https://www.frontiersin.org/articles/10.3389/fimmu.2022.994885/full