Homozygosity in any HLA locus is a risk factor for specific antibody production: the taboo concept 2.0
written by Henry Loeffler-Wirth; Claudia Lehmann, Nils Lachmann, Ilias Doxiadis,
INTRODUCTION
The molecules of the human leucocyte antigen system (HLA) play a pivotal role in immune recognition, and response. Their role in pregnancy, transfusion, and transplantation has been readily described (1). HLA molecules are receptor molecules for peptides presented to immune cells. Finally, they are the targets of immune response upon solid organ and stem cell transplantation. It is generally accepted that the formation of antibodies towards an allograft’s HLA leads to severe consequences for the graft and the patient (2). The number of individual alleles grows exponentially, reaching >38,000 to date (3). Methodologically, molecular typing by next-generation sequencing technology (NGS) and the bead-bound HLA molecules for antibody screening replaced earlier outdated techniques (4–7). Homozygosity can, on the one side, be considered as injurious in case of recessive genes, leading to diseases such as cystic fibrosis, endocrinological disorders, sickle cell anaemia, or other harmful mutations leading to incurable situations (8). On the other side, homozygosity can be beneficial, e.g., in rhesus factor compatibility, and in all unmutated genes, homozygosity is deemed positive (8). It is, however, still controversial whether homozygosity for any of the HLA loci is beneficial for the individual or the population (9–13). This information was retrieved using mostly low-resolution typing, with the exception of Hönger et al., who elaborated on the production of HLA antibodies in pregnancy. While several reports advocating that partner selection and mating are in favour of a still, presumably theoretical heterozygous advantage, field results do not support this hypothesis (14, 15). In viral infections such as HIV or SARS-CoV-2, homozygous patients are at risk compared to heterozygous individuals (16, 17). A proportion of patients on the national and international transplantation solid organ waitlists are homozygous for the HLA loci HLA-A, HLA-B, and HLA-DRB1, which are deemed transplantation relevant: in the allocation procedure, these patients receive special attention upon the availability of a suitable organ donor (18). Earlier studies showed that these patients accumulate on the waiting list and have a decreased graft survival rate and higher degree of sensitisation, which hampers the opportunity to be offered a suitable re-transplant (19). In general, the special attention to homozygous patients is restricted to mainly fully homozygous patients, as introduced in several organ procurement organisations. As a surrogate to transplantation results, we concentrate on the occurrence of specific antibodies in a subcohort of patients with a homozygosity on a single or multiple loci. Earlier, we reported that specific patient HLA combination led to a poorer graft survival, termed as the taboo concept (20). Here, we propose further development of that concept. The updated concept points to the probability of antibody production for single or multiple loci homozygous patients. In the present observational study, we concentrated on the occurrence of homozygosity of one or more of the eight HLA loci tested, and on their influence on the production of alloantibodies. These loci are HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQA1, HLA-DQB1, HLA-DPA1, and HLA-DPB1. We did not consider the loci HLA-DRB3, HLA-DRB4, and HLA-DRB5 because they are in tight linkage disequilibrium to and expressed in linkage with HLA-DRB1 genes.
OBJECTIVE
In a cooperative study of the University Hospital Leipzig, University of Leipzig, and the Charité Berlin on kidney transplant patients, we analysed the occurrence of HLA-specific antibodies with respect to the HLA setup of the patients. We aimed at the definition of specific HLA antigens towards which the patients produced these antibodies.
METHODS
Patients were typed for the relevant HLA determinants using mainly the next-generation technology. Antibody screening was performed by the state-of-the-art multiplex-based technology using microspheres coupled with the respective HLA alleles of HLA class I and II determinants.
RESULTS
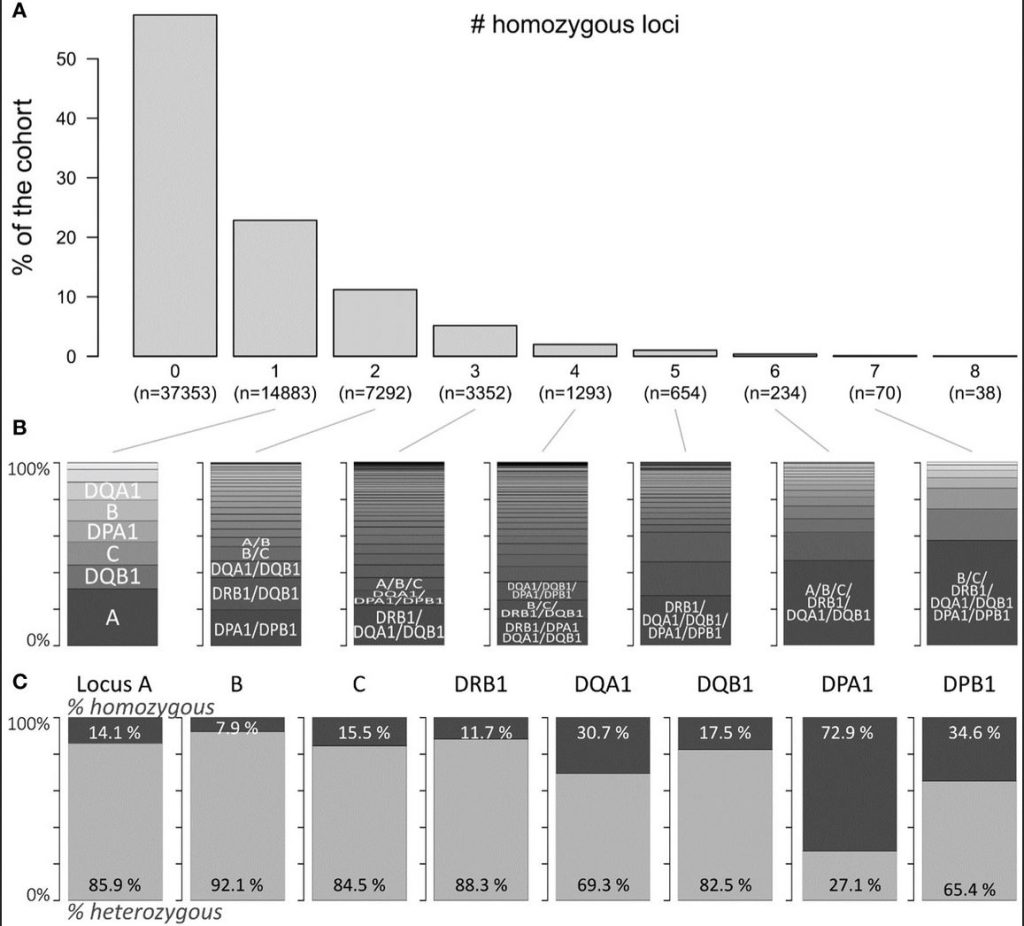
Patients homozygous for HLA-A*02, HLA-A*03, HLA-A*24, HLA-B*07, HLA-B*18, HLA-B*35, HLA-B*44, HLA-C*03, HLA-C*04, and HLA-C*07 in the class I group and HLA-DRB1*01, HLA-DRB1*03, HLA-DRB1*07, HLA-DRB1*15, HLA-DQA1*01, HLA-DQA1*05, HLA-DQB1*02, HLA-DQB1*03(7), HLA-DQB1*06, HLA-DPA1*01, and HLA-DPB1*04 in the class II group were found to have a significant higher antibody production compared to the heterozygous ones. In general, all HLA determinants are affected. Remarkably, HLA-A*24 homozygous patients can produce antibodies towards all HLA-A determinants, while HLA-B*18 homozygous ones make antibodies towards all HLA-B and selected HLA-A and C antigens, and are associated with an elevation of HLA-DRB1, parts of DQB1 and DPB1 alleles. Homozygosity for the HLA class II HLA-DRB1*01, and HLA-DRB1*15 seems to increase the risk for antibody responses against most of the HLA class I antigens (HLA-A, HLA-B, and HLA-C) in contrast to HLA-DQB1*03(7) where a lower risk towards few HLA-A and HLA-B alleles is found. The widely observed differential antibody response is therefore to be accounted to the patient’s HLA type.
CONCLUSIONS
Homozygous patients are at risk of producing HLA-specific antibodies hampering the outcome of transplantation. Including this information on the allocation procedure might reduce antibody-mediated immune reactivity and prevent graft loss in a patient at risk, increasing the life span of the transplanted organ.
. . .
to read the full article, click here: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1384823/full