Mechanism-based Multiscale Model to Dissect the Tipping Point from Liver Cirrhosis to Hepatocellular Carcinoma
A favourable prognosis for HCC patients is limited by the fact that curative treatment options depend on early detection. However, the currently available methods are not sensitive enough to ensure this. Robust and reliable methods to identify patients at very early stages of tumour development would both significantly increase the window of opportunity for curative treatment options and open up as well as avenues for novel therapeutic interventions.
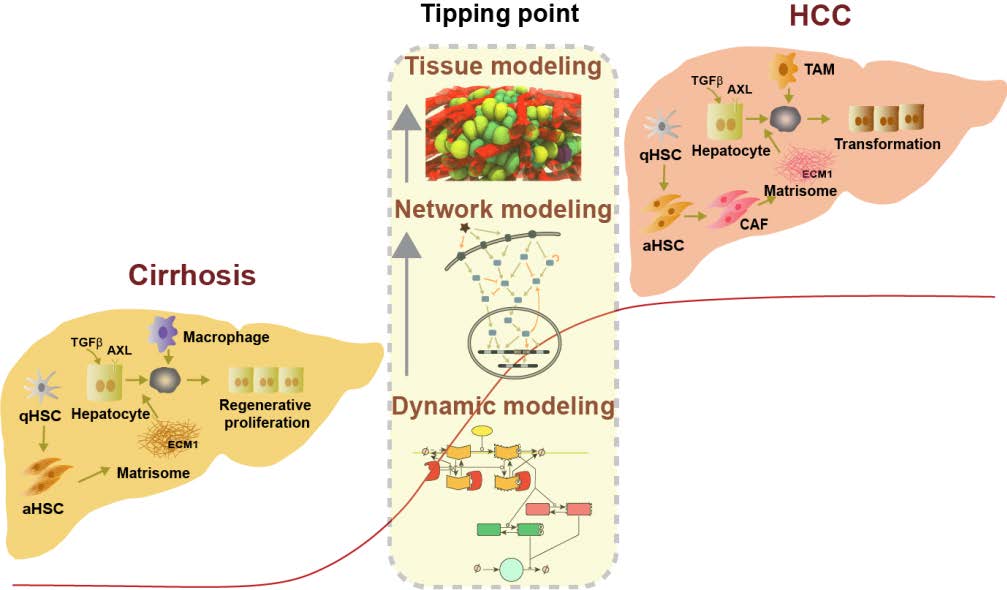
Optimal would be a non-invasive set of tools to identify patients with chronic liver liver disease who are at high risk of developing HCC. The development of liver cirrhosis is a common risk factor for the development of HCC. Within the framework project will use a systems medicine approach (non-invasive) to obtain measurable tissue and cell parameters in patients with chronic inflammatory liver disease and liver cirrhosis, which are indicative for exceeding the tipping point (TIP) from liver cirrhosis to HCC. In this context, the TIP is defined as the stage of liver cirrhosis in which minor changes in cellular and tissue factors create a microenvironment cellular and tissue factors create a microenvironment that is responsible for driving the tissue reaction towards malignant transformation and cancer cell development.
Critical tissue and Cell parameters include structural changes in cirrhotic liver architecture, including changes in cellular phenotypes, particularly hepatic stellate cells (HSCs), stellate cells (HSC), macrophages and hepatocytes, qualitative and quantitative changes in the matrisome and alterations in the TGFβ signalling pathway. In addition longitudinal clinical data from very large national and European patient cohorts, some of which have already been collected in LiSyM. Imaging-Omics and dynamic pathway data will be used to support the mathematical models developed in LiSyM mathematical models developed in LiSyM at the tissue, cellular and molecular scales, and thus define the patient-specific TIP in the cirrhotic regenerative node. A graphical summary of the C-TIP-HCC network is shown in Fig1.
Technically, a mechanistic spatially and temporally resolved multiscale model is being based on the above-mentioned tissue and cell parameters, which is capable of the specific risk of a single patient with cirrhosis by parameter measurement and model simulation cirrhosis to exceed the TIP for tumourigenesis. Given the contextual complexity, we propose the use of a model that integrates quantitative data on the pathogenesis of TIP transgression integrated. These focus on aberrant signalling pathways and matrisomal dynamics affecting on parenchymal and stromal cells. The model will be able to identify at-risk patients much earlier than is possible with current clinical approaches, which mainly focus on the early detection of established HCC.
We assume that our approach will enable early detection („even before“ the TIP) patients at (highest) risk into a programme of innovative interventions (in terms of measurable parameters) at the precancerous stage, in order to prevent or significantly delay the crossing of TIP.
Project Aim
The ultimate goal of the C-TIP-HCC is a „mechanistic multiscale model to describe dynamic changes in regenerative nodes across a tipping point (TIP) towards development in patients with cirrhosis to facilitate early monitoring and intervention“ and facilitate intervention“. To achieve this, we will conduct in-depth studies on cirrhotic regenerative nodes.
project leader at IZBI Dr. Stephan Hoehme
duration 01.07.2021 – 30.06.2024
joint project / third-party founding
coordinator: Prof. Dr. rer. nat. Steven Dooley, Sektion Molekulare Hepatologie, II. Medizinische Klinik, Medizinische Fakultät Mannheim, Universität Heidelberg